- Is enthalpy positive in an exothermic reaction?
- Is ΔH for an exothermic reaction is positive?
- Is negative change in enthalpy exothermic?
- Is enthalpy positive or negative?
- When ΔH is positive the reaction is select and when ΔH is negative the reaction is?
- What is positive enthalpy?
- What is the formula for an exothermic reaction?
- What is an example of exothermic change?
Is enthalpy positive in an exothermic reaction?
So, if a reaction releases more energy than it absorbs, the reaction is exothermic and enthalpy will be negative. Think of this as an amount of heat leaving (or being subtracted from) the reaction. If a reaction absorbs or uses more energy than it releases, the reaction is endothermic, and enthalpy will be positive.
Is the energy change for an exothermic reaction positive or negative?
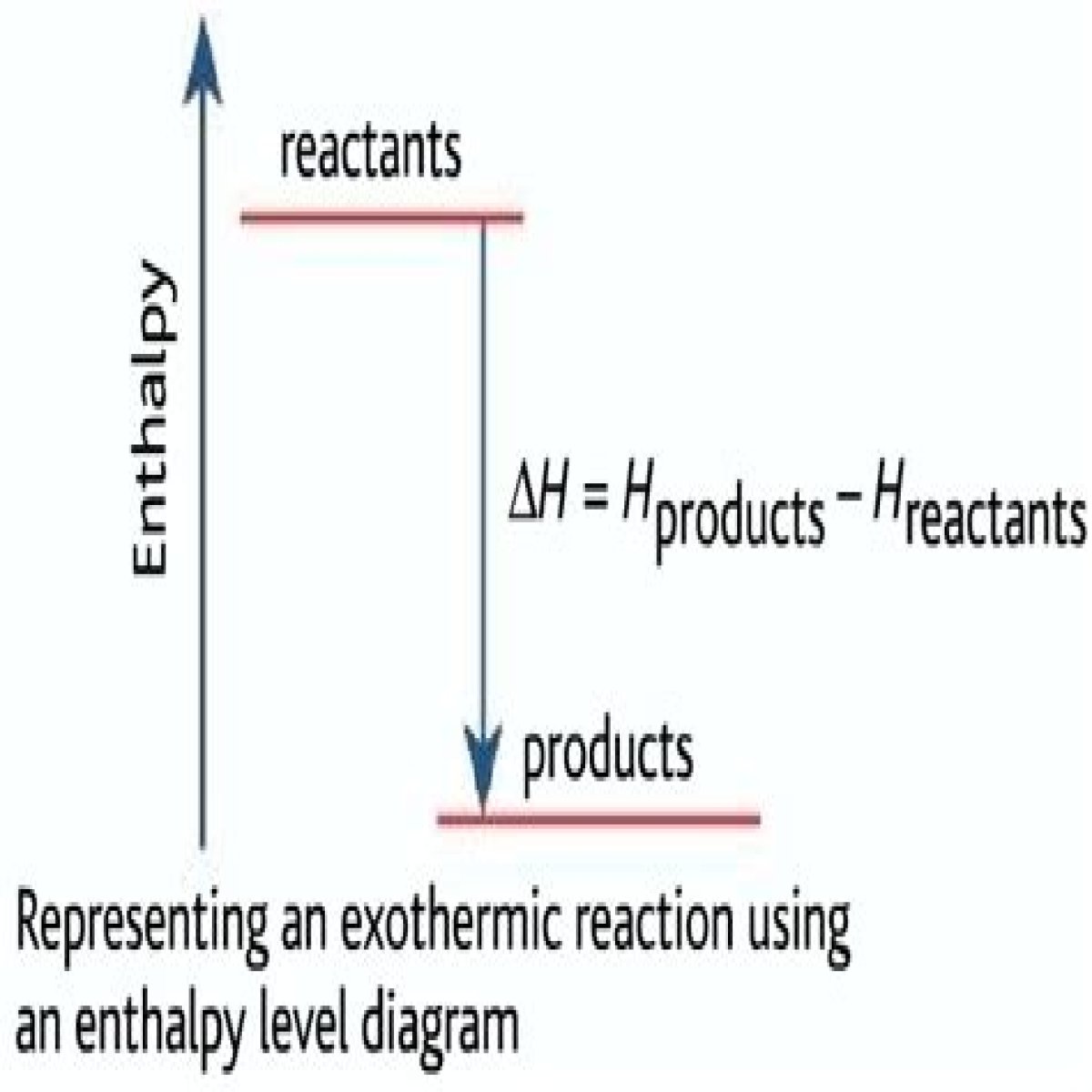
enthalpy changeIn an exothermic reaction, the products are at a lower energy than the reactants. The difference between the energy of the reactants and the energy of the products is called the enthalpy change (∆H) of the reaction. For an exothermic reaction, the enthalpy change is always negative.
Is ΔH for an exothermic reaction is positive?
A system that releases heat to the surroundings, an exothermic reaction, has a negative ΔH by convention, because the enthalpy of the products is lower than the enthalpy of the reactants of the system.
Is enthalpy change positive or negative?
Explanation: The change in enthalpy in an exothermic reaction is negative, since overall heat is lost ( “exo”thermic means that heat is leaving).
Is negative change in enthalpy exothermic?
Exothermic Reactions In an exothermic reaction, energy is released because the total energy of the products is less than the total energy of the reactants. For this reason, the change in enthalpy, ΔH , for an exothermic reaction will always be negative.
Is enthalpy change positive for endothermic reaction?
In an endothermic reaction, the products are at a higher energy than the reactants. This means that the enthalpy change of the reaction (∆H) is positive.
Is enthalpy positive or negative?
Chemists routinely measure changes in enthalpy of chemical systems as reactants are converted into products. If so, the reaction is endothermic and the enthalpy change is positive. If more energy is produced in bond formation than that needed for bond breaking, the reaction is exothermic and the enthalpy is negative.
Which statement is true when ΔH is negative?
When energy is transferred as heat from the system to the surroundings, ΔH is negative. A combustion reaction is exothermic.
When ΔH is positive the reaction is select and when ΔH is negative the reaction is?
Using Delta H They can only measure changes in enthalpy. When enthalpy is positive and delta H is greater than zero, this means that a system absorbed heat. This is called an endothermic reaction. When enthalpy is negative and delta H is less than zero, this means that a system released heat.
Is change in enthalpy always positive?
As such, the change in enthalpy for an endothermic reaction is always positive.
What is positive enthalpy?
3. What does it mean if Enthalpy is POSITIVE or NEGATIVE? A positive ∆H means that a reaction is endothermic as heat is absorbed from the surroundings to the system and the surroundings feel cold as the temperature decreases.
Is enthalpy change of combustion negative?
The enthalpy change of combustion will always have a negative value, of course, because burning always releases heat.
What is the formula for an exothermic reaction?
In general, exothermic reactions may be represented by the following exothermic reaction equation. With A+B being the Reagent and C + D the Product of the Reaction. A + B → C + D + q (heat energy) Energy is required to break chemical bonds which means that energy is spent or used up.
What are some examples of endothermic and exothermic reactions?
Examples of exothermic processes include burning of coal, rust formation and dissolution of quick lime in water. On the other hand, some endothermic processes include dissolution of Ammonium Chloride in water and nitric oxide formation. In chemical reactions, energy is required in the breaking up of atomic bonds.
What is an example of exothermic change?
Chemistry at About.com states that most exothermic reactions, unlike phase changes, result in higher entropy in the products. An example of an exothermic reaction that is not a phase change is combustion.
Is endothermic exothermic or endothermic?
Endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. A good example of an endothermic reaction is photosynthesis. Combustion is an example of an exothermic reaction. The categorization of a reaction as endo- or exothermic depends on the net heat transfer.