Is SP sp2 or sp3 more acidic?
If we’re saying that an sp hybridized atom is more acidic than something sp2 which is more acidic than something sp3, we can say that acidity goes to the left from sp3 being the least acidic, sp being the most acidic.
Is sp3 more basic than sp2?
Hybridization on the N also affects basicity. An increase in s character on an atom increases the electronegativity of that atom which favors acidity and therefore disfavors basicity. Hence sp3-hybridized nitrogen is more basic than either sp2 or sp hybridized nitrogen.
Which is stronger SP sp2 sp3?
A bond between sp3 and sp2 is stronger than a bond between sp3 and sp3 because sp2 hybridized orbitals contains 33.33% s-character while sp3 contains 25% s-character. A general rule, the more s-character in a hybridized orbital, the stronger a bond it will form.
Does acidity increase with hybridization?
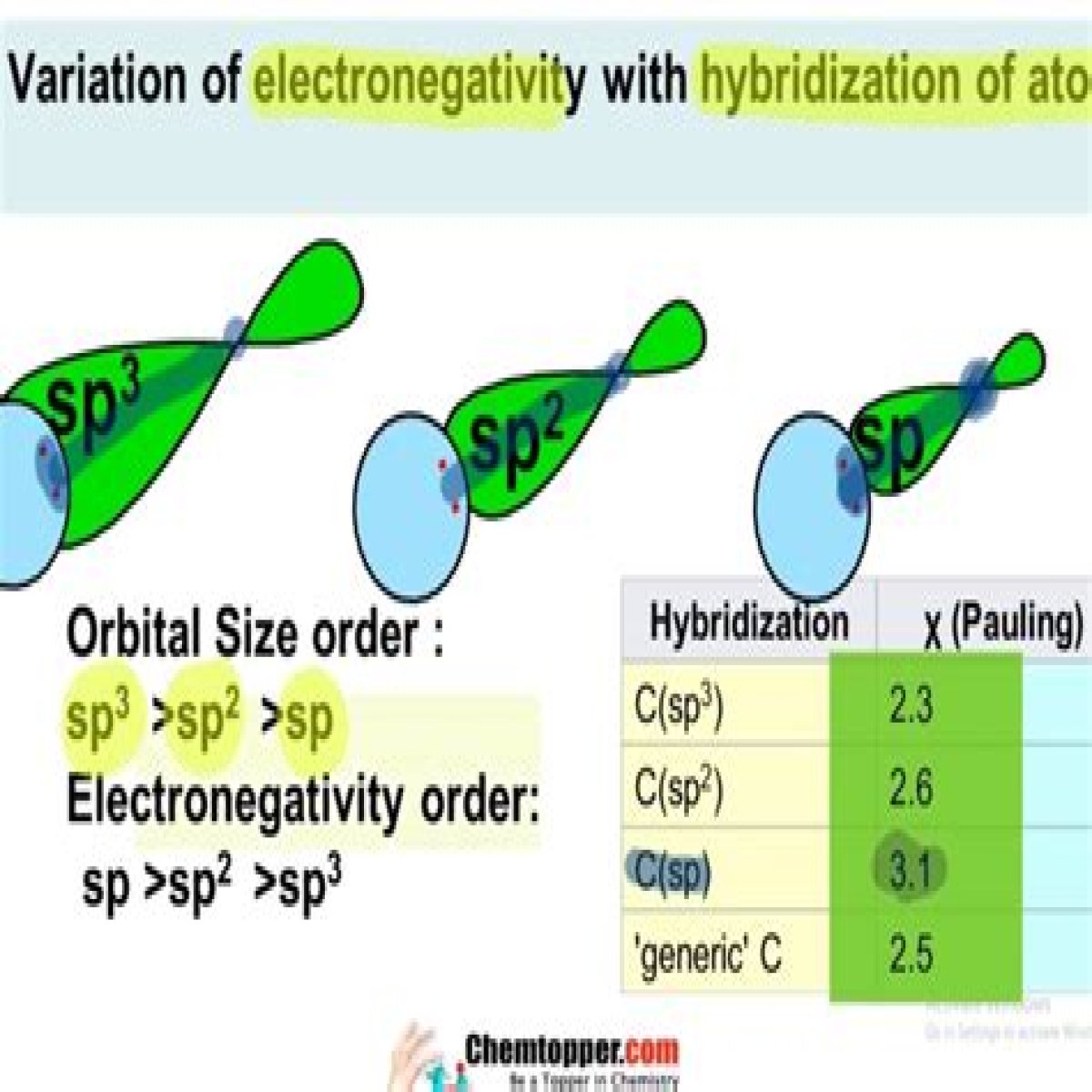
hybridization of the carbon. We know that s orbitals are closer to the nucleus than p orbitals. This tells us that the more s character a hybrid orbital has the closer are the electrons to the atom. Such atoms are less willing to share their electron density and thereby have greater acidic character.
Why are SP hydrogens acidic?
An sp hybridized atom though is still weakly acidic and therefore would require a relatively strong base to deprotonate it. In this case though, it’s more acidic than say an sp2 or sp3 hybridized atom because the conjugate base is more stabilized on an sp hybridized atom than an sp2 or sp3 hybridized atom.
Is SP most acidic?
Since s orbital is closest to the nucleus, thus electron which is present in orbitals with more s character will be more attracted by the nucleus and as a result electronegativity of sp is greater than the other two. Therefore sp is more acidic followed by sp2 and sp3.
Is SP or sp3 more stable?
Just like any other atomic orbital, each sp3 hybrid orbital can house 2 elections. S-character and the stability of the anion: The closer the electrons are to the nucleus, the more stable they are. Therefore, when bearing the negative charge, sp3 species are less stable than sp2 and sp species.
Is SP stronger than sp3?
sp3 = 25% s-character, 75% p-character sp2 = 33% s-character, 66% p-character sp = 50% s-character, 50% p-character Therefore, sp hybrid orbital have more s-character results in the formation of more stronger bonds.
What is more reactive sp2 or sp3 carbon?
Sp is more reactive than sp2 and Sp3,Sp2 is more reactive than Sp3. In Sp,one sigma and two pi bond, pi bond is less stable mean more reactive then sigma bond. While in Sp3 no pi bond is present, it has only sigma bond which are more stable and less reactive.
Which hybridization produces the strongest bond among alkane alkene and alkyne?
Alkynes are more reactive than alkenes. The reactivity is: Alkenes > Alkynes > Alkanes. Alkenes have one pi-bond between two (or more) carbon atoms, along with a sp2-sp2 hybridised orbital bonding (sigma bond).
Why is sp3 more acidic?
Electronegativity of the depends upon s character. Since s orbital is closest to the nucleus, thus electron which is present in orbitals with more s character will be more attracted by the nucleus and as a result electronegativity of sp is greater than the other two. Therefore sp is more acidic followed by sp2 and sp3.
How do you calculate acidity formula?
Find the size of the base of the atom as compared to the others. Larger atoms are closer to the bottom of the periodic table, while smaller ones are closer to the top. Compare the differences in molecular structure. The closer the negative ion is to the H+ ion in the molecule, the stronger the acid is.