- Which family contains the most reactive elements?
- Which group of elements are very reactive?
- What is reactive family?
- Which two families of elements are most reactive and why?
- In Which family would you expect to find an element that is very reactive with water and whose atoms have one valence electron?
- Which is the most reactive?
- What is an element that lacks most of the properties of metals?
- What do all members of a family of elements have in common?
Which family contains the most reactive elements?
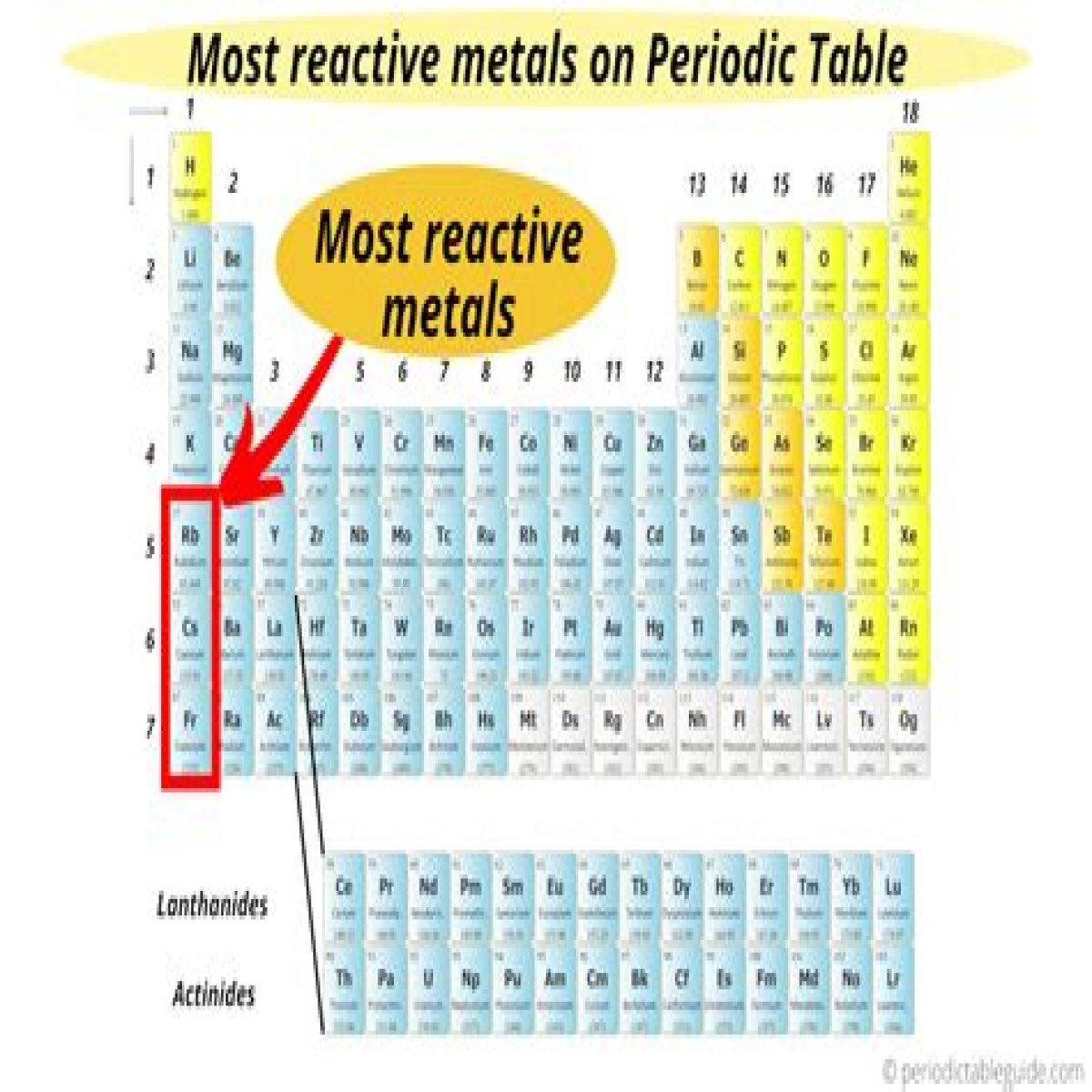
alkali metalsThe family of elements that contains the most reactive metals are considered alkali metals. Alkali metals are comprised of: Francium. Sodium.
Which group of elements are very reactive?
Group 1 of the periodic table includes hydrogen and the alkali metals. Because they have just one valence electron, group 1 elements are very reactive. As a result, they are found in nature only in combination with other elements. Alkali metals are all solids at room temperature.
What are very reactive elements?
The halogens, alkali metals, and alkaline earth metals are highly reactive.
- The most reactive element is fluorine, the first element in the halogen group.
- The most reactive metal is francium, the last alkali metal (and most expensive element).
- The least reactive elements are the noble gases.
What family of metals is most reactive?
Alkali metalsAlkali metals are among the most reactive metals. This is due in part to their larger atomic radii and low ionization energies. They tend to donate their electrons in reactions and have an oxidation state of +1.
What is reactive family?
The most reactive metals in the periodic tables are the alkali metals, followed by the alkaline earth metals.
Which two families of elements are most reactive and why?
Hydrogen is a very reactive gas, and the alkali metals are even more reactive. In fact, they are the most reactive metals and, along with the elements in group 17, are the most reactive of all elements.
Which families are highly reactive which family is unreactive?
Group 7A (or 17) elements are also called halogens. This group contains very reactive nonmetal elements. The noble gases are in group 8A. These elements also have similar properties to each other, the most significant property being that they are extremely unreactive, rarely forming compounds.
Which element is most reactive element?
Cesium is second from the bottom of this group, has 6 shells of electrons, and it matches the features of a reactive atom, making it the most reactive element.
In Which family would you expect to find an element that is very reactive with water and whose atoms have one valence electron?
Group 1 of the periodic table includes hydrogen and the alkali metals. Because they have just one valence electron, group 1 elements are very reactive.
Which is the most reactive?
What is an element family?
In chemistry, a group (also known as a family) is a column of elements in the periodic table of the chemical elements. There are 18 numbered groups in the periodic table; the f-block columns (between groups 2 and 3) are not numbered.
What family of elements is relatively unreactive and why?
What family of elements is relatively unreactive and why? The noble gases because their valence shell is has 8 electrons. Many chemical reactions take place because atoms don’t have a full outer/valence electron shell.
What is an element that lacks most of the properties of metals?
An element that lacks most of the properties of a metal. diatomic molecule. A molecule consisting of two atoms. halogen. Group 17; a family of very reactive elements whose atoms gain or share one electron. noble gas. Group 18; a family of unreactive elements whose atoms do not gain, loose, or share valence electrons.
What do all members of a family of elements have in common?
All the members of a family of elements have the same number of valence electrons and similar chemical properties. The horizontal rows on the periodic table are called periods. Group (family): A vertical column in the periodic table.
What family of elements are highlighted on the periodic table?
The highlighted elements of the periodic table belong to the alkali metal element family. The alkali metals are recognized as a group and family of elements. These elements are metals. Sodium and potassium are examples of elements in this family.