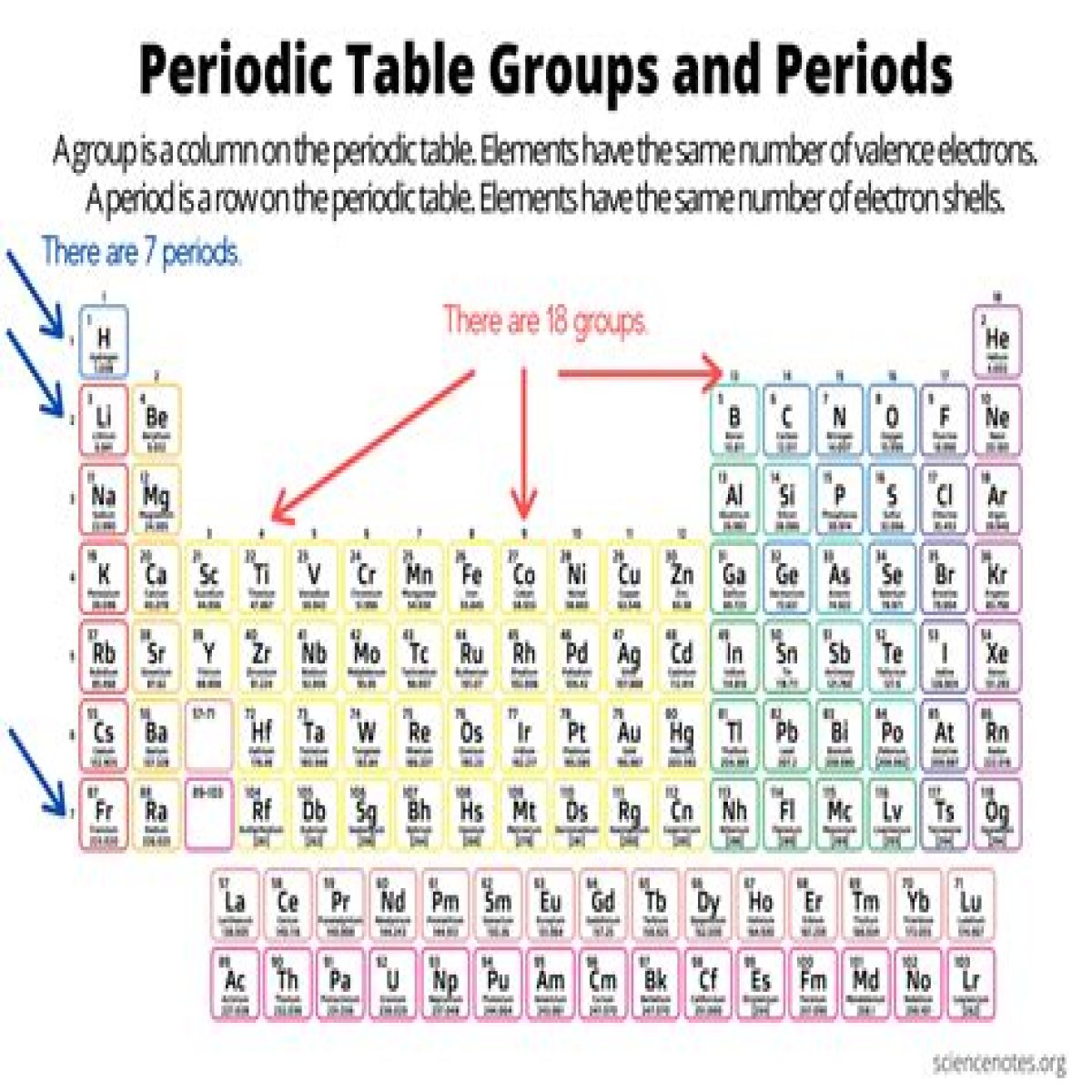
Groups and periods are two ways of categorizing elements in the periodic table. Periods are horizontal rows (across) the periodic table, while groups are vertical columns (down) the table. Atomic number increases as you move down a group or across a period.
Table of Contents
Element Groups
Elements in a group share a common number of valence electrons. For example, all of the elements in the alkaline earth group have a valence of two. Elements belonging to a group typically share several common properties.
The groups in the periodic table go by a variety of different names: